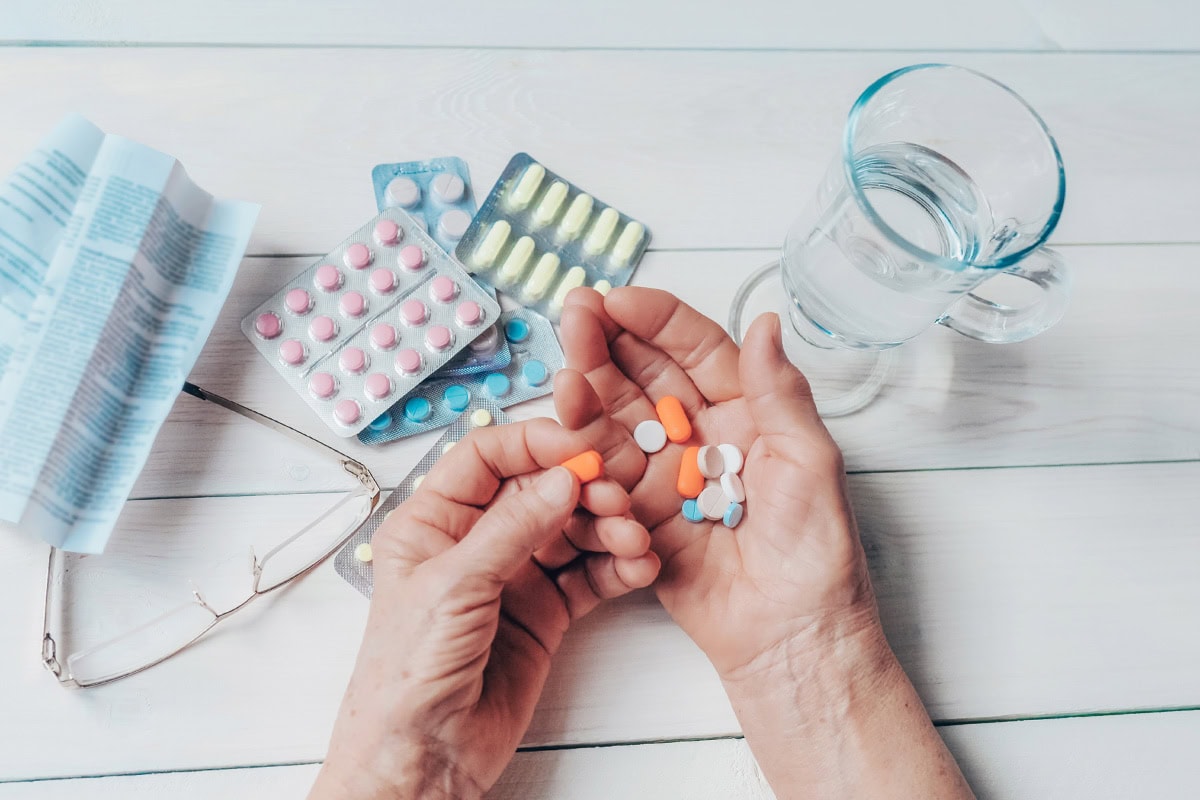
Injured by a Defective Drug or Medical Device?
Get started with a free case evaluation. No Fee Until We Win.
100% Free Evaluation
Provide a few details and our team will handle the rest.
"*" indicates required fields
When a drug or medical device you trusted causes serious harm instead of healing, you need more than just any lawyer—you need a battle-tested sword and shield fighting for your rights against billion-dollar corporations
At Keller & Keller, we’ve been maximizing client settlements and delivering justice since 1936, and we’re not backing down from Big Pharma or medical device manufacturers. With over 80 years of experience taking on the pharmaceutical and medical device industries’ giants, we’ve recovered millions of dollars for clients harmed by defective products. That’s not just a number—that’s families who got justice when companies put profits over people.
We’re a family-run firm, and we treat you like family because we understand that behind every case is a real person who trusted a medication or medical device and got hurt. We reach out and make sure our clients feel like they can have a cup of coffee with us. No fancy legal jargon, no runaround—just straight talk about what we’re going to do to hold these companies accountable.
Why You Need a Defective Drug And Device Lawyer
When a medication or medical device harms you instead of helps you, the manufacturers have already started protecting themselves. These aren’t small operations—they’re billion-dollar corporations with armies of lawyers whose job is to minimize what they pay out. You need someone on your side who knows how to fight back.
Navigating Complex Legal Claims
Understanding Insurance Company Tactics
Understanding Statute of Limitations
What to Do After a Drug or Device Injury
If you’ve been harmed by a defective drug or medical device, these steps can protect both your health and your legal claim.
See What Your Case Is WorthOnly after consulting with your doctor about safe alternatives or next steps
Get evaluated for drug or device-related injuries even if symptoms seem minor
Keep the medication bottle, packaging, inserts, device components, and any documentation
Write down what you’re experiencing and when it started
Save all bills, test results, and doctor’s notes related to your injury
Manufacturers may contact you—don’t sign releases or settlements without legal advice
File an adverse event report to help protect others from similar harm
The sooner we start investigating, the stronger your case becomes

The Keller & Keller Approach:
Injury Lawyers Who Fight to Win
From the moment you contact us, you’ll notice the difference in our approach. We treat you like family because we are family-run. Our client-first philosophy means we take the time to understand not just the details of your accident but how it has affected your life, your work, your family, and your future.
We understand that being injured is stressful enough without having to chase down your lawyer for updates. That’s why we’ve built our practice around transparent, consistent communication. When you hire us, you’ll have direct access to the attorney handling your case as well as a dedicated paralegal who monitors its daily status. We keep you informed throughout every stage of your claim, explaining what’s happening, what to expect next, and how it affects your case.
With over 80 years of experience fighting accident victims, we’ve built a reputation as battle-tested advocates who know how to win. Our attorneys have successfully handled thousands of cases. We’ve gone toe-to-toe with the biggest insurance companies and won. Our courtroom experience means we’re always prepared to take your case to trial if that’s what it takes to secure fair compensation. This willingness to fight—and our track record of success—often motivates insurance companies to offer better settlements to avoid facing us in court.
The Defective Drug And Device Claim Process with Keller & Keller
When you hire us, we immediately start building your case because time matters in pharmaceutical and medical device litigation.
Types of Defective Drug And Device Cases We Handle
We represent clients harmed by all types of dangerous and defective prescription medications and medical devices.
If the drug or device that injured you isn’t listed above, call us with the product name. We can quickly determine whether it’s a potential claim our network can handle.
The Hard Truth
Defective Drug And Device Statistics That Demand Action
The pharmaceutical and medical device industries’ track records show why we need to hold them accountable.
Studies show 6.7% of hospitalized patients suffer serious adverse drug reactions. This makes ADRs the 4th leading cause of death—ahead of diabetes, pneumonia, and car accidents.
This means the true number of injuries is far higher than reported. Even though serious adverse event reports doubled from 2006 to 2014, most victims never report their injuries, leaving dangerous products on the market and putting more lives at risk.
These activities occurred between 2003 and 2016, totaling $33 billion. From 1991 to 2021, drug makers paid $62.3 billion in penalties across 482 cases. Despite these massive fines, many companies treat penalties as just another business expense and continue putting profits over safety.
Common Causes of Drug And Device Injuries
Understanding how drugs and devices become defective helps establish liability in your case.
Drug And Device Injuries We Represent
Defective medications and medical devices can cause devastating injuries that change lives forever.

What Sets Keller & Keller Apart
With so many law firms advertising their services, why choose Keller & Keller? The differences are clear.
No Fee Until We Win!
Get Started TodayDon’t Take Our Word For It!
See what our clients have to say about their experience with Keller & Keller.
View More Testimonials“Jim Keller is the real deal.”
Gerry
One of the more impressive things about Jim Keller, the personal injury lawyer, besides the outstanding results he obtains for his clients, is his dedication to his clients and their causes. I judge a man’s qualities by how I see him act, not by what he tells me he does. I judge by how he operates over time. Jim Keller is the real deal.
Take Action Now
Your Defective Drug And Device Claim Starts Here
Free Consultation
We’ll evaluate your case honestly and explain your options in plain English. We’ll come to you—whether you’re at home, in the hospital, or wherever is convenient.
We Fight
Our experienced team fights to investigate thoroughly, deal with insurance companies, and handle all the legal complexities while you focus on getting better.
You Recover
Focus on healing while we handle the rest. We’ll fight for you like we’d fight for our own family, because that’s exactly how we see you.
Zero Fee Guarantee
We believe everyone deserves quality legal representation, regardless of their financial situation. That’s why we work on a contingency basis—you pay absolutely nothing unless and until we win your case. No upfront costs, no hidden fees, no financial risk to you.
Our Story
Family Tradition
Frequently Asked Questions
Have questions about your case? We’re here to help